What is 21 CFR Part 11?
Title 21, Part 11 of the U.S. Food and Drug Administration’s (FDA) Code of Federal Regulations is a guideline for using electronic records and electronic signatures. The code requires drug makers, medical device manufacturers, biotech companies, biologics developers and other FDA-regulated industries (except food manufacturers) to implement controls; including audits, validation systems, and documentation. This includes software and systems involved in processing many forms of data as part of business operations and product development.[1] Whether you are a food and beverage producer, chemical manufacturer, or a process manufacturer compliance of 21 CFR Part 11 compliance is critical.
Why we need 21 CFR Part 11
The regulation was created to ensure companies implement good business practices to maintain the trustworthiness, reliability, and integrity of electronic records and to ensure that the authenticity of electronic records would be equivalent to paper records when submitted. All companies and industries that submit or utilize electronic records and signatures regulated by the FDA must comply with this federal regulation.
Compliance with regulatory requirements is a business-critical need that must be maintained, and the FDA recognizes that a technically advanced software solution can help companies manage compliance. Specifically, CFR 21 Part 11 states that enterprise resource planning (ERP) systems must provide:
Extensive transaction audit functionality with field, user, time and date reference, Document signature printing association for technical or quality assurance generated reports, Digital signature to support field and/or screen level security authentication with change reason codes, and, access verification and historical user, time and date references.
When considering business management solutions, there are several areas you need to consider for compliance.
- Do the features on your computer system properly manage electronic records and processes?
- Do your current Standard Operating Procedures (SOPs) address the IT infrastructure requirements?
- Are you using your ERP solution as it is intended? Do you have controls in place to identify when the system doesn’t function as per its intended use?
Sage X3 has the following functionality, to help companies adhere to 21 CFR Part 11:
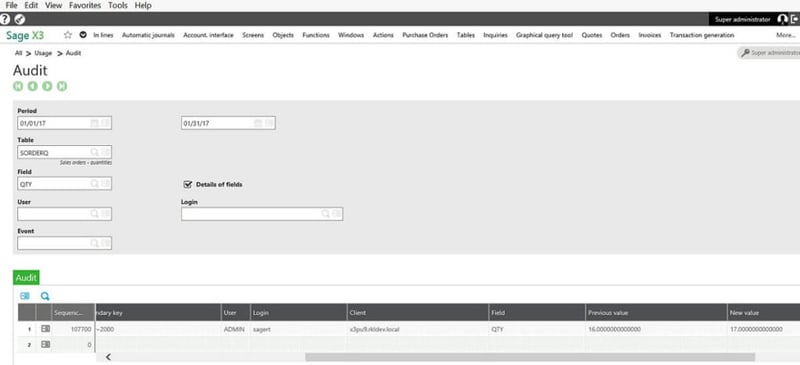
Audit Trails - Associated with the creation, modification, and deletion of electronic records, audit trails are now standard in Sage X3. The functionality records user name, date, time, previous data, new data and the reason for the change.
Digital Electronic Signatures - An electronic signature framework includes tables, programs, actions and objects to store, configure and collect unique e-signatures, which are permanently linked to the object and cannot be modified or copied.
Document Signatures - Documents requiring handwritten signatures, such as Certificates of Analysis or Technical Sheets, are generated with an image linked to the specific document. The image plate is controlled and linked to the user profile.
Validation Scripts - Documentation describing various process controls deployed by Sage is available. These scripts are flexible in design, associated with clearly identified and documented procedures. They are easily transferred or incorporated into custom validation and cGMP documents to support company initiatives.
Security Features - Several security standards safeguard against unauthorized use, including automatic logoff after a period of inactivity, auto logout after too many failed logon attempts and logging of all user activity. [2]
For detailed information about how Sage X3 helps companies comply with each requirement of Part 11, read the whitepaper Managing 21 CFR Part 11 Compliance X3.
While it may seem that complying with Part 11 is a burdensome, costly undertaking, adhering to the regulations yields several benefits, including:
- Reductions in system vulnerability and abuse,
- Lower compliance-driven costs,
- Shorter validation time,
- Reductions in entry errors,
- Lower costs related to record retention,
- Improved data integration and modeling capabilities,
- Advanced search capability via a decision support system and data warehouse, and
- Increased speed of information exchange.

Want to Make 21 CFR Part 11 Compliance Easier?

Click below and learn how Sage X3 delivers all the industry-specific functionality a food and beverage producer needs to take the manual tracking and hassle our of the FDA compliance process.
[1]See Guidance for Industry Part 11, Electronic Records; Electronic Signatures – Scope and Application (http://www.fda.gov/RegulatoryInformation/Guidances/ucm125067.htm)
[2] https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11


